Consolidation of Analytical Activities for a New Water Microbiology Laboratory
Regulatory Compliance and Accreditation
General requirements for the competence of testing and calibration laboratories. This standard has been developed to promote confidence in the operation of laboratories. It contains requirements that allow laboratories to demonstrate that they operate competently and have the capability to generate valid results.
The ISO 17025 standard has become the international reference standard for testing and calibration laboratories that want to demonstrate their ability to obtain reliable results and develop management systems for their quality, administrative, and technical activities.
01. Context
A company in the Testing, Inspection, and Certification (TIC) sector has three laboratories in a specific region, located at significant distances from each other. Two of these laboratories specialize in the investigation and remediation of contaminated sites, soil analysis, waste characterization, acceptance criteria testing for waste, wastewater effluent analysis, and landfill monitoring. The third laboratory focuses on analyzing physico-chemical and microbiological parameters in water, providing analytical services to companies in the water hygiene, water treatment, and hospital sectors, with a strong specialization in the detection of Legionella.
All three laboratories are accredited according to ISO 17025 and collectively have a broad accreditation scope in both microbiological and physico-chemical fields for different matrices.
A year and a half ago, for logistical and commercial strategy reasons, the company decided to establish a new microbiology laboratory within one of the contaminated soil laboratories. This decision aimed to reduce the need to transport samples to the water laboratory and simultaneously diversify the analytical services of the soil laboratory towards water microbiology, providing more local support for microbiological analytical services in that region.
After the design and construction phases of the facilities and their equipment, the company proceeded with the implementation of methods, validation, and staff training, with support from the water laboratory, which had the necessary experience for this implementation.
The new laboratory started with four staff members, and the initial accreditation scope requested from the accreditation body included total coliforms, Escherichia coli, Enterococci, aerobic bacterial count at 37ºC and 22ºC, Clostridium perfringens, Pseudomonas spp. and Pseudomonas aeruginosa, Legionella spp., and Legionella pneumophila, both by culture and qPCR.
With everything prepared, the company applied for ISO 17025 accreditation for the new laboratory. After the audit in the summer of 2023, the accreditation body expressed concerns about the weak implementation of the quality system and, primarily, the lack of experience of the staff, which compromised the technical competence of the laboratory for the analytical activities included in the requested accreditation scope.
02. Challenge
“The challenge of the project was clear.”
“Consolidate the laboratory’s analytical activities to acquire the necessary technical competence to manage the laboratory and achieve ISO 17025 accreditation, all in the shortest possible time.”
03. Approach
An action plan was prepared with three clear objectives:
- Consolidate the laboratory’s activities and the implemented quality system.
- Implement an accelerated training program for both technical and laboratory management.
- Directly supervise the analytical activities conducted during the project’s execution.
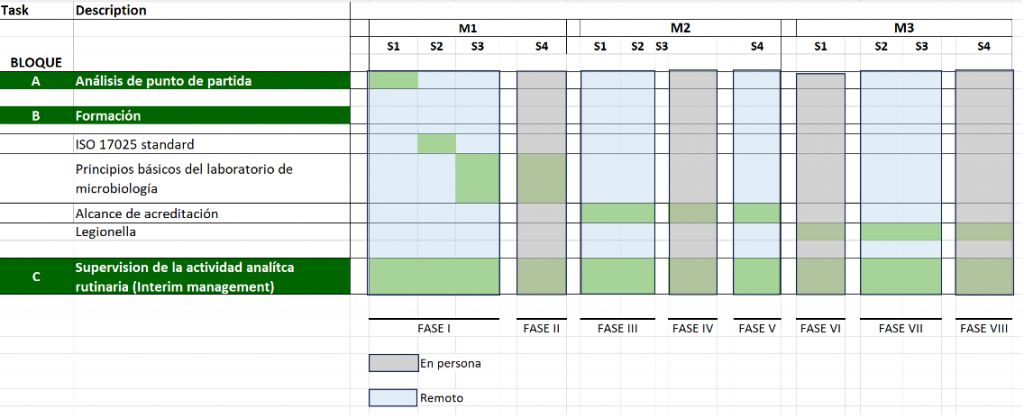
The project was organized into eight phases over a total duration of three months, with both remote and on-site activities.
As shown in Figure 1, three sets of actions were identified:
- Initial Assessment: Evaluating the maturity of the quality system, the methods used and their validations, and assessing the technical competencies of the staff.
- Staff Training: Focused on deep knowledge of ISO 17025, the basic principles of a microbiology laboratory, the methods included in the accreditation scope, and specific training on Legionella.
- Monitoring and Supervision: Detailed tracking of all activities performed in the laboratory, combined with practical daily training for the staff.
Remote activities were carried out over eight weeks, consisting of two sessions per week, each lasting two hours. Additionally, in case of emergencies, the consultant was contacted by phone for immediate resolution.
Alongside the remote activities, the consultant interspersed four weeks of physical presence in the laboratory. This allowed for the consolidation of actions performed remotely and detailed monitoring of the project’s progress and the subsequent consolidation of the laboratory.
04. Results
Once the project was completed, an internal audit was conducted, followed by a request for an extraordinary audit by the accreditation body, which was successfully passed without any issues, ultimately achieving the accreditation.
Currently, the business managed by the laboratory is around €400,000 annually, with a growth perspective of 14% per year. Additionally, there are plans to expand the accreditation scope soon to further increase the business volume.
As a result, the management of this project allowed the company to:
- Achieve ISO 17025 accreditation in just three months.
- Consolidate the laboratory’s analytical activities, forming a technically solid and competent team.
- Focus internal resources from the other water laboratory on their daily analytical activities and business development.
- Integrate the new laboratory’s activities with the existing one, homogenizing and making processes more efficient.



